Lucitanib NLT 98%
Product Number : MC504575
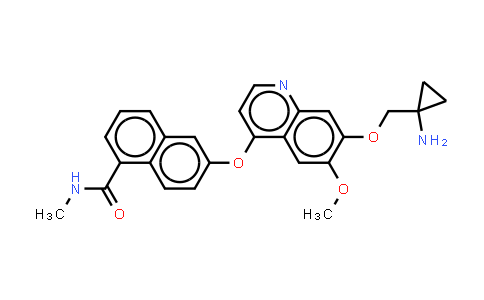
CAS Number : 1058137-23-7
Molecular Formula : C26H25N3O4 | Molecular Weight : 443.49
Synonyms : E-3810
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
| MolCore specializes in manufacturing high-purity CAS No.1058137-23-7, Lucitanib with the molecular formula C26H25N3O4 and molecular weight 443.49 delivering critical API intermediates for global pharmaceutical and research industries, certified under ISO quality systems. | |
* The above information is for reference only.
| Chemical Name | Lucitanib |
|---|---|
| CAS Number | 1058137-23-7 |
| MDL Number | MFCD20527751 |
| Molecular Formula | C26H25N3O4 |
| Molecular Weight | 443.49 |
| Synonyms | E-3810 |
Lucitanib (E-3810) is a novel dual inhibitor of VEGFR and FGFR, potently and selectively inhibits VEGFR1, VEGFR2, VEGFR3, FGFR1 and FGFR2 with IC50s of 7 nM, 25 nM, 10 nM, 17.5 nM, and 82.5 nM, respectively. IC50 & Target: IC50: 7 nM (VEGFR1), 25 nM (VEGFR2), 10 nM (VEGFR3), 17.5 nM (FGFR1), 82.5 nM (FGFR2), 5 nM (CSF-1R)[1] In Vitro: Consistent with the inhibitory activity of VEGFR and FGFR auto-phosphorylation, Lucitanib potently inhibits VEGF and bFGF-stimulated HUVEC proliferation with IC50 of 40 and 50 nM, respectively. Besides, Lucitanib (E-3810) also inhibits CSF-1R with IC50 of 5 nM[1]. Lucitanib potently inhibits FGFR2 activity (Ki<0.05 μM), follows by PDGFRα activity (Ki=0.11 μM). The Ki values obtained for DDR2, LYN, CARDIAK, CSBP (2), EPHA2, and YES range between 0.26 and 8 μM[2]. In Vivo: Lucitanib (E-3810), at oral dosing of 20 mg/kg for 7 consecutive days, completely inhibits (P<0.01) the bFGF induced angiogenic response compare with the response in vehicle-treated mice. Lucitanib (E-3810) shows a broad spectrum of activity, being active in all the xenografts tested (HT29 colon carcinoma, A2780 ovarian carcinoma, A498, SN12K1, and RXF393 renal carcinomas) with dose-dependent inhibition of tumor growth. E-3810 significantly delays growth during treatment, but tumors resume their growth when treatment is suspended; in a few cases, tumor regression is observed[1]. The activity of Lucitanib (E-3810) given at the doses of 15 mg/kg is tested on MDA-MB-231 breast cancer transplanted subcutaneously, at a late stage, when tumor masses reach 350 to 400 mg. This tumor xenograft is very sensitive to Lucitanib (E-3810), with complete tumor stabilization lasting throughout the 30-day treatment. As in other tumor models, tumors re-grow after withdrawal of Lucitanib (E-3810) at a rate similar to control tumors[3].
© Copyright 2015-2025 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
