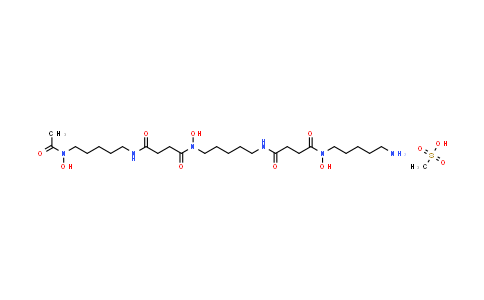
Deferoxamine (mesylate) NLT 98%
Product Number : MC520761
CAS Number : 138-14-7
Molecular Formula : C26H52N6O11S | Molecular Weight : 656.79
Synonyms : Desferrioxamine B mesylate;DFOM
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
| MolCore specializes in manufacturing high-purity CAS No.138-14-7, Deferoxamine (mesylate) with the molecular formula C26H52N6O11S and molecular weight 656.79 delivering critical API intermediates for global pharmaceutical and research industries, certified under ISO quality systems. | |
* The above information is for reference only.
| Chemical Name | Deferoxamine (mesylate) |
|---|---|
| CAS Number | 138-14-7 |
| MDL Number | MFCD00058605 |
| Molecular Formula | C26H52N6O11S |
| Molecular Weight | 656.79 |
| Synonyms | Desferrioxamine B mesylate;DFOM |
Deferoxamine mesylate is an iron chelator, and effectively reduces oxidative stress and neuronal death. In Vitro: Deferoxamine treatment significantly increases HIF-1α binding under all culture conditions, including hypoxic and high-glucose. The mechanism of deferoxamine is through improving HIF-1α biological function through scavenging oxygen free radicals[1]. Deferoxamine (5 μM) has significant effect on the tumor-associated stromal cells cellular multiplication, and cells die at day 7 after exposure to 50 μM and 100 μM deferoxamine. Deferoxamine (5 μM-100 μM) inhibits the proliferation of BMMSCs, and induces apoptosis of MSCs in a dose-dependent manner. Deferoxamine influences the expression of adhesion proteins on MSCs[3]. Deferoxamine (30, 60, 120 μM) shows lower expression of HIF-1α in a concentration dependent way in AdMSCs[4]. In Vivo: Deferoxamine (100 mg/kg, i.p.) lowers the mortality rate of subarachnoid hemorrhage (SAH) rat. Deferoxamine (100 mg/kg, i.p.) attenuates Evan’s blue extravasation in cortex, ameliorates the tight junction detachment and preserves the integrity of the base membrane examined in electron microscope at day 3 after SAH. Deferoxamine attenuates degradation of BBB proteins after SAH and significantly reduces ferritin expression at day 3 in the cortex, and improves neurologic behavior and cognitive deficits after experimental[1]. Ten µL of 1 mM deferoxamine-treated wounds display significantly accelerated healing from day 7 onward and heal significantly faster than control-treated wounds in diabetic mice. Deferoxamine-treated wounds and dimethyloxalylglycine-treated wounds heal significantly faster than control-treated wounds in aged mice[2]. In deferoxamine (10 mg/mL)-treated TG mice, there is a decrease in both soluble and insoluble Aβ40 and Aβ42. Both pGSK3β and β-catenin are significantly increased by approximately 50% in the deferoxamine-treated mice[5].
© Copyright 2015-2025 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
