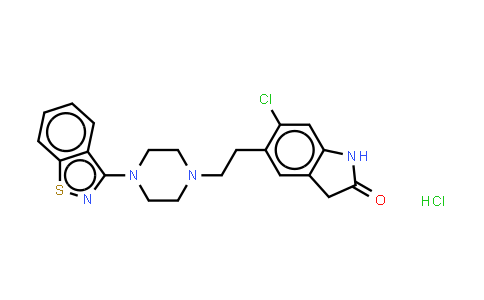
Ziprasidone (hydrochloride monohydrate) NLT 98%
SKU : MC521179
CAS Number : 138982-67-9
Molecular Formula : C21H22Cl2N4OS | Molecular Weight : 449.40
Synonyms : CP 88059 (hydrochloride monohydrate)
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
* The above information is for reference only.
* If the product has intellectual property rights, a license granted is must or contact us.
| Chemical Name | Ziprasidone (hydrochloride monohydrate) |
|---|---|
| CAS Number | 138982-67-9 |
| MDL Number | MFCD06795476 |
| Molecular Formula | C21H22Cl2N4OS |
| Molecular Weight | 449.40 |
| Synonyms | CP 88059 (hydrochloride monohydrate) |
Ziprasidone hydrochloride monohydrate (CP 88059 hydrochloride monohydrate) is a combined 5-HT (serotonin) and dopamine receptor antagonist which exhibits potent effects of antipsychotic activity. Target: 5-HT receptor; Dopamine receptor Ziprasidone hydrochloride monohydrate is the salt form of ziprasidone, which possesses an in vitro 5-HT2A/dopamine D2 receptor affinity ratio higher than any clinically available antipsychotic agent. In vivo, Ziprasidone hydrochloride monohydrate antagonizes 5-HT2A receptor-induced head twitch with 6-fold higher potency than for blockade of d-amphetamine-induced hyperactivity, a measure of central dopamine D2 receptor antagonism. Ziprasidone hydrochloride monohydrate also has high affinity for the 5-HT1A, 5-HT1D and 5-HT2C receptor subtypes, which may further enhance its therapeutic potential [1]. Ziprasidone hydrochloride monohydrate sulfoxide and sulfone were the major metabolites in human serum. The affinities of the sulfoxide and sulfone metabolites for 5-HT2 and D2 receptors are low with respect to Ziprasidone hydrochloride monohydrate, and are thus unlikely to contribute to its antipsychotic effects [2]. Ziprasidone hydrochloride monohydrate was associated with significant differential adverse effects relative to placebo in BPM, BPD, and schizophrenia with no significant difference in weight gain in all 3 groups. Self-reported somnolence was increased across the 3 conditions. Subjects with BPM were more vulnerable to EPS than those with BPD or schizophrenia [3]. Clinical indications: Bipolar I disorder; Bipolar disorder; Mania; Schizophrenia FDA Approved Date: February 2001
Related Products
© Copyright 2015-2024 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
