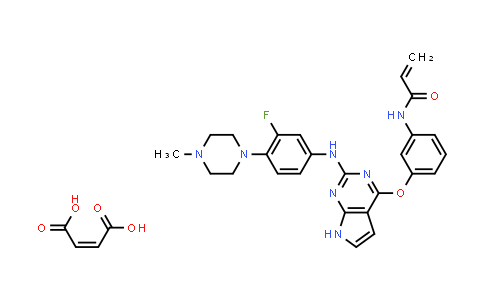
Avitinib (maleate) NLT 98%
SKU : MC527414
CAS Number : 1557268-88-8
Molecular Formula : C30H30FN7O6 | Molecular Weight : 603.60
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
* The above information is for reference only.
* If the product has intellectual property rights, a license granted is must or contact us.
| Chemical Name | Avitinib (maleate) |
|---|---|
| CAS Number | 1557268-88-8 |
| Molecular Formula | C30H30FN7O6 |
| Molecular Weight | 603.60 |
Avitinib maleate is a pyrrolopyrimidine-based irreversible epidermal growth factor receptor (EGFR) inhibitor with an IC50 of 7.68 nM. IC50 & Target: IC50: 7.68 nM (wild-type EGFR)[1] In Vitro: Avitinib is structurally distinct from previously reported pyrimidine-based irreversible EGFR inhibitors such as osimertinib and rociletinib. Avitinib is designed specifically to inhibit EGFR active mutations and the T790M acquired resistant mutation, while sparing wild type EGFR. Avitinib selectively inhibits EGFR active and T790M mutations with up to 298-fold increase in potency compared to wild-type EGFR. Avitinib exhibits potent inhibitory activity with IC50 value of 0.18 nM against EGFR L858R/T790M double mutations, nearly 43-fold greater potency over wild-type EGFR (IC50=7.68 nM). Avitinib selectively inhibits mutant EGFR phosphorylation with IC50 values of 7.3 nM and 2.8 nM in NCI-H1975 and NIH/3T3_TC32T8 cells, about 115- and 298-fold more sensitive than that of the inhibition of wild type EGFR in A431[1]. In Vivo: Oral administration of avitinib at daily dose of 500 mg/kg results in complete remission of tumors with EGFR active and T790M mutations for over 143 days with no weight loss. Three major metabolites of avitinib are tested and show no wild-type EGFR inhibition and off-target effects such as inhibition of IGF-1R. Avitinib is safe in non-small cell lung cancer (NSCLC) patients at the dose range between 50 mg and 550 mg once per day and no hyperglycemia and other severe adverse effects are detected such as grade 3 QT prolongation[1].
Related Products
© Copyright 2015-2024 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
