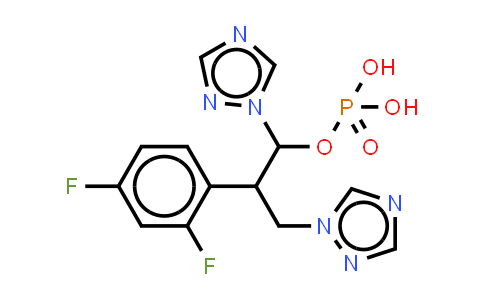
Fosfluconazole NLT 98%
SKU : MC536467
CAS Number : 194798-83-9
Molecular Formula : C13H13F2N6O4P | Molecular Weight : 386.25
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
* The above information is for reference only.
* If the product has intellectual property rights, a license granted is must or contact us.
| Chemical Name | Fosfluconazole |
|---|---|
| CAS Number | 194798-83-9 |
| MDL Number | MFCD08277022 |
| Molecular Formula | C13H13F2N6O4P |
| Molecular Weight | 386.25 |
Fosfluconazole is a prodrug of Fluconazole that is widely used as an antifungal agent. IC50 & Target: Antifungal[1] In Vitro: To investigate the polarized bioconversion and the Transwell transport of phosphate prodrugs in Caco-2 monolayer, 10 μM Fosfluconazole or Fosphenytoin is dosed either in the apical or basal compartment in Transwell plates. Both prodrugs are efficiently cleaved in the apical compartment after a 2 h incubation. To further investigate the kinetics of ALP-mediated bioconversion, the concentration-dependent ALP-mediated bioconversions are conducted to determine the Michaelis-Menten constant (Km) of prodrug bioconversion in Caco-2 monolayers. The saturation curves of Fosphenytoin and Fosfluconazole with the concentration increase are found. The estimated Km values of Fosphenytoin and Fosfluconazole are 1160 and 357 μM, respectively[2]. In Vivo: The apparent half-life for Fosfluconazole bioconversion in intestinal mucosa scraps is 10 min[2]. Fluconazole (FLCZ) is an antifungal agent that is efficacious in the treatment of fungal peritonitis. Fosfluconazole (F-FLCZ) is the phosphate prodrug of FLCZ, which is highly soluble compared with FLCZ. F-FLCZ is useful against fungal peritonitis in continuous ambulatory peritoneal dialysis (CAPD) patients because it has a high water solubility. The aims of the present study are to characterize the peritoneal permeability of FLCZ and the pharmacokinetics of FLCZ and F-FLCZ after intraperitoneal (i.p.) administration to peritoneal dialysis rats. FLCZ or F-FLCZ is administered intravenously and intraperitoneally. After the i.p. administration of F-FLCZ, FLCZ is detected in circulating blood and the dialyzing fluid in peritoneal dialysis rats. The concentration of plasma FLCZ after the i.p. F-FLCZ administration is lower than that after the intravenous (i.v.) F-FLCZ administration. It is considered that the dose should be increased appropriately when F-FLCZ is administered intraperitoneally. The profiles of plasma FLCZ after i.v. and i.p. administrations are analyzed using a two-compartment model in which the distribution volume of the peripheral compartment is fixed at a volume of the dialyzing fluid (peritoneal dialysis PK model). The peritoneal dialysis PK model could describe the profiles of plasma and dialyzing fluid FLCZ. These results suggest that FLCZ and F-FLCZ could be administered intraperitoneally for the treatment of fungal peritonitis in CAPD patients[3].
Related Products
© Copyright 2015-2024 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
