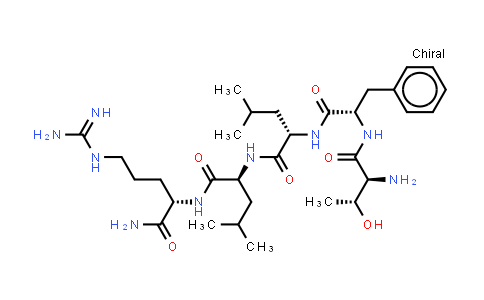
TFLLR-NH2 NLT 98%
SKU : MC537099
CAS Number : 197794-83-5
Molecular Formula : C31H53N9O6 | Molecular Weight : 647.81
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
* The above information is for reference only.
* If the product has intellectual property rights, a license granted is must or contact us.
| Chemical Name | TFLLR-NH2 |
|---|---|
| CAS Number | 197794-83-5 |
| MDL Number | MFCD03093514 |
| Molecular Formula | C31H53N9O6 |
| Molecular Weight | 647.81 |
TFLLR-NH2 is a selective PAR1 agonist with an EC50 of 1.9 μM. Sequence: Thr-Phe-Leu-Leu-Arg-NH2. IC50 & Target: EC50: 1.9 μM (PAR1)[1] In Vitro: PAR1 agonists stimulate concentration-dependent increases in [Ca2+]i and in the proportions of neurones. The maximal increase in [Ca2+]i above basal is detected in response to 10 μm TF-NH2(peak 196.5±20.4 nM, n=25) when 50–80% of identified neurones responded[1]. SW620 cells cultured in the supernatant of TFLLR-NH2-activated platelets upregulate E-cadherin expression and downregulate the vimentin expression. In the in vitro platelet culture system, a TFLLR-NH2 dose-dependent increase of secreted TGF-β1 is detected in the supernatant[2]. In Vivo: Injection of TF-NH2 into the rat paw stimulates a marked and sustained oedema. An NK1R antagonist and ablation of sensory nerves with capsaicin inhibit oedema by 44% at 1 h and completely by 5 h. In wild-type but not PAR1−/− mice, TF-NH2 stimulates Evans blue extravasation in the bladder, oesophagus, stomach, intestine and pancreas by 2–8 fold. Extravasation in the bladder, oesophagus and stomach is abolished by an NK1R antagonist[1]. TFp-NH2 produces notable contraction at 3-50 μM and relaxation at 0.3-50 μM, in the absence of apamin. The concentration-response curve for TFp-NH2-induced contraction is remarkably shifted left, when the TFp-NH2-induced relaxation is blocked by apamin at 0.1 μM[3].
Related Products
© Copyright 2015-2024 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
