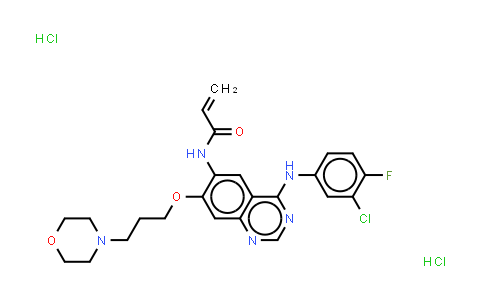
Canertinib (dihydrochloride) NLT 98%
SKU : MC546607
CAS Number : 289499-45-2
Molecular Formula : C24H27Cl3FN5O3 | Molecular Weight : 558.86
Synonyms : CI-1033 dihydrochloride;PD-183805 dihydrochloride
Quote Request| Purity | NLT 98% |
|---|---|
| Storage | at 20ºC 2 years |
* The above information is for reference only.
* If the product has intellectual property rights, a license granted is must or contact us.
| Chemical Name | Canertinib (dihydrochloride) |
|---|---|
| CAS Number | 289499-45-2 |
| MDL Number | MFCD09954112 |
| Molecular Formula | C24H27Cl3FN5O3 |
| Molecular Weight | 558.86 |
| Synonyms | CI-1033 dihydrochloride;PD-183805 dihydrochloride |
Canertinib dihydrochloride (CI-1033 dihydrochloride) is a potent and irreversible EGFR inhibitor; inhibits cellular EGFR and ErbB2 autophosphorylation with IC50s of 7.4 and 9 nM. IC50 & Target: IC50: 7.4 nM (EGFR), 9 nM (ErbB2)[1] In Vitro: Canertinib dihydrochloride (CI-1033 dihydrochloride) significantly inhibits growth of cultured melanoma cells, RaH3 and RaH5, in a dose-dependent manner. IC50 is approximately 0.8 μM and by 5μM both cell lines are completely growth-arrested within 72 h of treatment. Incubation of exponentially growing RaH3 and RaH5 with 1 μM canertinib accumulated the cells in the G1-phase of the cell cycle within 24 h of treatment without induction of apoptosis. 1 μM canertinib inhibits ErbB1-3 receptor phosphorylation with a concomitant decrease of Akt-, Erk1/2- and Stat3 activity in both cell lines[2]. In Vivo: Canertinib dihydrochloride (CI-1033 dihydrochloride) shows superior in vivo antitumor activity, giving growth delays in A431 xenografts exceeding 50 days following oral administration[1]. The growth of human malignant melanoma xenografts, RaH3 and RaH5, in nude mice is significantly inhibited by i.p. injections of 40 mg/kg/day canertinib (Fig. 4). The anti-proliferative effect on melanoma xenografts is visible already within 4 days of treatment and further increased throughout the treatment period as observed through the differences in tumor volumes, reaching statistical significance within 18 days of treatment[2].
Related Products
© Copyright 2015-2024 Hangzhou MolCore BioPharmatech Co.,Ltd. All rights reserved.
